My wife watches the Food Network a lot and I occasionally watch it with her but I can only take so much of it before I go off and read or work on one of my projects. I’ve noticed however in the various cooking contests that sometimes a chef will deconstruct a familiar recipe. This more or less means they break the recipe down into its components and present them as separate pieces or perhaps by putting what goes inside on the outside instead.
I’ve discussed the DLCO test with numerous people and have found that many know and understand (or at least remember) the ATS/ERS criteria for test quality. At the same time however, there seems to be very few people that understand the formula used to calculate the single-breath DLCO and I suspect this is probably because most of us didn’t like the mathematics classes we had to attend in high school or college (and tried to forget what we learned as quickly as we could afterwards).
The DLCO formula isn’t that complicated however, and more importantly all the components of the DLCO test and the reasons for the ATS/ERS quality criteria are embedded within it. All this seems to be a good reason to de-construct the DLCO “recipe” and try to explain it’s various pieces.
As a reminder the single-breath DLCO formula is:
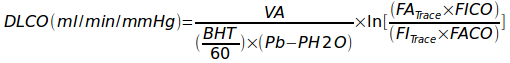
Where:
VA = alveolar volume in ml
BHT = breath holding time in seconds
Pb = barometric pressure
PH2O = partial pressure of water vapor in the lung
FITrace = fractional concentration of tracer gas in the inspired DLCO mixture
FATrace = fractional concentration of tracer gas in the alveolar sample
FICO = fractional concentration of CO in the inspired DLCO mixture
FACO = fractional concentration of CO in the alveolar sample
I think the part that bothers everybody the most is:
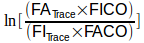
and that’s because there’s two different things going on here. First, the part within the brackets:
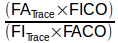
is intended to correct the initial CO concentration for the dilution that occurs when the DLCO test gas mixture is inhaled and mixes with the gas that was within the lung at the start of the inhalation. The whole point of the DLCO test is to measure CO uptake but the initial concentration for this measurement is not what’s in the tank, it’s what’s in the lungs after it has been diluted by the lung’s residual volume and deadspace gas.
This might be easier to understand if it was re-stated as:
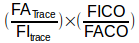
where the relationship between the exhaled and inhaled concentrations of the tracer gas:

is what says how much the DLCO gas mixture was diluted, and
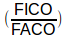
is the part that says how much CO was taken up.
FITrace and FICO are more or less constants. For this reason it’s easy to see the lower FATrace is, the more the inhaled gas was diluted. FACO however, is affected both by dilution and CO uptake and should therefore always be proportionally lower than FATrace.
So, what’s a tracer gas? In this particular instance it’s any gas that is highly insoluble (being inert helps too but it’s not a requirement). There’s nothing in the alveolar-capillary membrane that acts as a barrier to the movement of gases and in fact that’s what it’s optimized for. The amount of any gas that can be absorbed by blood and tissue however, is based on how soluble they are. Gases like helium, argon, neon and methane are highly insoluble so when they are inhaled only a tiny amount is absorbed by blood and tissue. This means that when a gas mixture containing an insoluble gas is inhaled and exhaled, the ratio between the inhaled and exhaled concentrations indicates how much it was diluted. This is the basis for the Helium Dilution FRC test and for measuring VA.
One of the interesting things about combining the tracer gas and CO in the same equation:
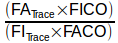
is that the accuracy of the CO and tracer gas analyzers becomes unimportant as long as the analyzers are linear and their zero level is accurate. This is because it is the ratio of concentrations that matters, not what the concentrations actually are. For this reason, the actual concentration of CO and tracer gas that comes from a tank is not terribly important. The choice of 0.3% CO was fairly arbitrary and the accuracy of single-breath DLCO tests using CO concentrations from 3.0% to 0.03% are not significantly different from those performed at 0.3%. What matters is patient safety and the ability of the gas analyzer to provide a signal that’s adequate to differentiate between adjacent concentrations across it’s entire range.
The Ln (natural logarithm) part of the equation outside the brackets
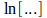
is there because rate at which CO is taken up by the lung is not linear and in fact decreases over time. Once CO uptake starts (which is soon as the DLCO gas mixture clears the deadspace of the airways and reaches and alveoli) the concentration of CO decreases. As the CO concentration in the lung decreases, the rate at which it crosses into the blood stream also decreases which looks something like this:
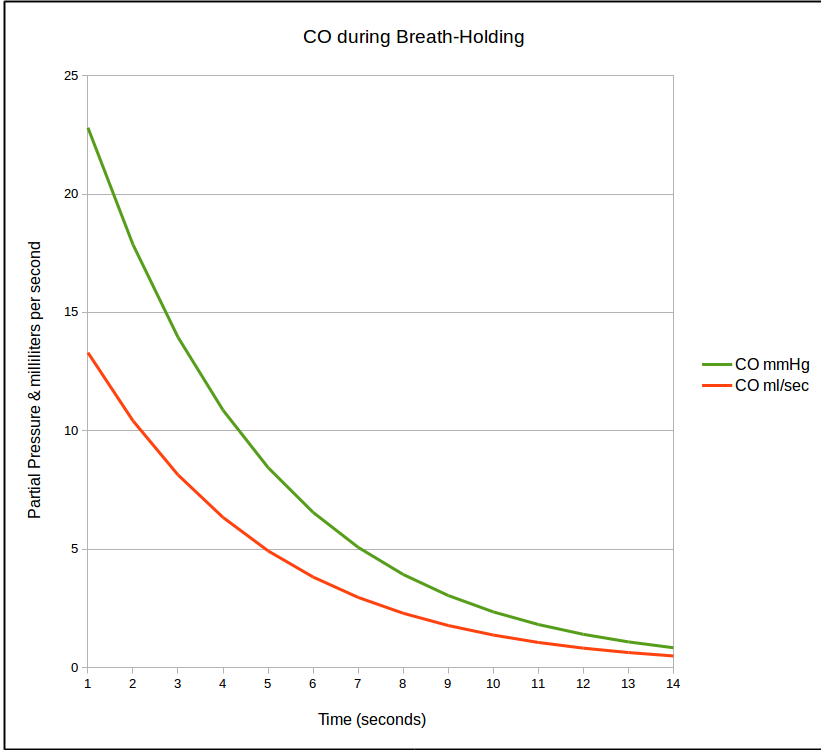
This decline follows an exponential curve and when it is re-stated as a natural logarithm it becomes a straight line.
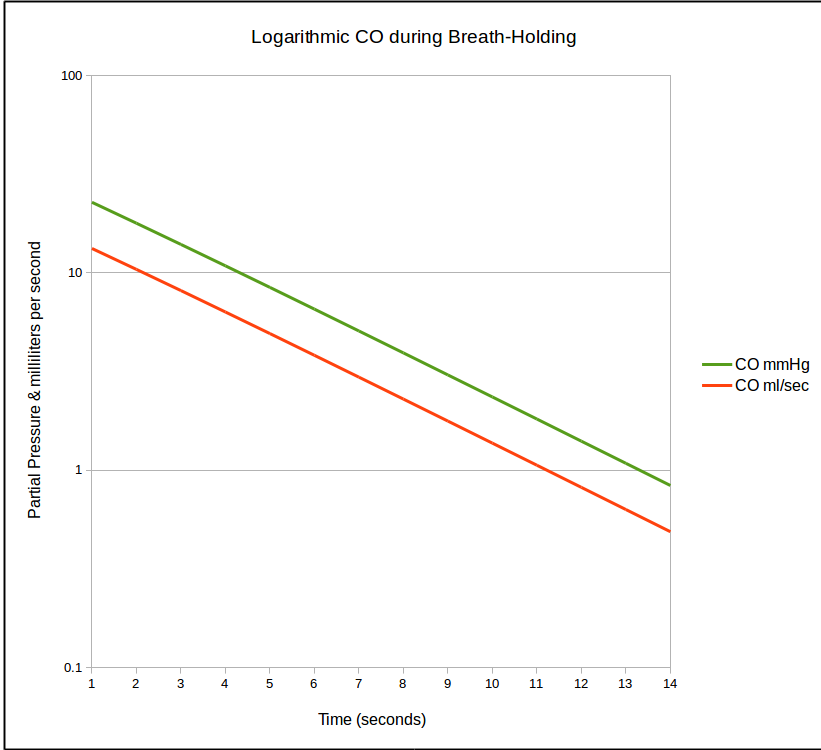
So basically, this section of the equation:
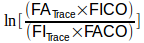
determines the rate at which CO declines in the lung during breath-holding, with an adjustment for the dilution that occurs when the gas mixture is inhaled.
On the left upper side of the equation, VA is the lung volume “seen” by the DLCO gas mixture. Ignoring for the moment the 2017 ERS/ATS DLCO standard’s recommendations that VA should be calculated using a mass balance equation over the entire exhalation since this has yet to be implemented on any test systems, it is calculated separately as:
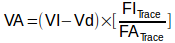
Where:
VA = alveolar volume (ml)
VI = inspired volume (ml)
Vd = machine + anatomical deadspace.
The purpose of VA is to convert the rate at which CO disappears given by the last section of the formula into a flow rate. Remember that DLCO is expressed (at least in part) as a flow rate, i.e. ml/min. The rate at which CO disappears in any given lung during breath-holding (given by the right side of the equation) can be the same for large, small or even diseased lungs so by itself it is not terribly informative. When it is expressed as a flow rate however, it can be related to normal values for a given gender, height and age.
On the lower left side of the equation, BHT, Breath-Holding Time, which is in seconds, is divided by 60:
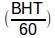
in order to convert BHT to fractional minutes. This wouldn’t be necessary if DLCO was expressed as ml/sec but it’s not, so the conversion from seconds to fractional minutes is needed.
BHT however, is always going to be something of an approximation. This is because the DLCO formula basically assumes that inspiration and expiration are instantaneous. Because they aren’t, there are several ways in which BHT is routinely measured.
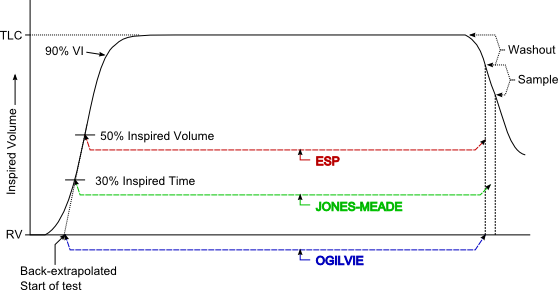
The current 2017 and the 2005 ERS/ATS DLCO standards recommends using the Jones-Meade approach to measuring BHT and this is mostly because J-M makes some consideration for the prolonged expiratory times (and alveolar sample collection times) for individuals with COPD.
Finally,
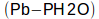
obviously corrects for the partial pressure of water vapor in the lung but less obviously, Pb essentially converts the fractional concentrations of CO and the tracer gas into their corresponding partial pressures. Remember that rate that CO disappears in the lung:
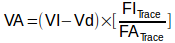
is based on fractional concentrations, not partial pressures. DLCO is expressed as ml per minute per mmHg and the pressure component comes from the barometric pressure.
When looking at the raw data for a DLCO test it may be helpful to remember that FICO, FITrace, Pb and PH2O are essentially constants. In addition, even though BHT is not a constant, it’s usually within a very narrow range and given that it’s divided by 60 it doesn’t have a large effect on test-to-test changes. This basically means that everything on the lower left side of the DLCO equation changes very little from test-to-test.
VA, on the other hand is a function of VI and FATRACE and these can and do change a fair amount between tests. As long as the VI ends more or less at TLC however, these differences tend to cancel out and VA should be fairly constant between tests. A large difference in VA from one test to another indicates a large difference in test quality. In general the DLCO test with a larger VA should probably be considered more accurate than one with a smaller VA, however VA can be falsely skewed both upwards and downwards when VI is suboptimal (due to inhomegenous distribution of the inhaled gas mixture).
It’s hardest to characterize test-to-test changes in FACO since it is a function of both FATrace and the actual CO transfer rate in the lung during the breath-holding period. Although significant changes in FACO can point to test quality issues, determining the actual cause is more difficult.
When a chef deconstructs a recipe it’s successful sometimes and sometimes it isn’t but this is often due to how well the individual components were treated. Deconstructing the single-breath DLCO calculation not only helps to highlight its components but also shows that understanding it isn’t as formidable as it first appears. We may not feel comfortable with the math we routinely use (whether we realize it or not since pretty much everything is done automatically by our computer systems) but deconstructing equations is a good way to understand what’s important in the tests we perform.