The ATS/ERS recommendation for assessing the response to bronchodilator is based solely on changes in FEV1 or FVC. An FEV1 that does not improve significantly following bronchodilator inhalation is considered to be one of the hallmarks of COPD. Many individuals with COPD however, can have symptomatic relief and an improvement in their exercise capacity without a significant post-bronchodilator increase in FEV1. This means that FEV1 may not be the only criteria for assessing bronchodilator response.
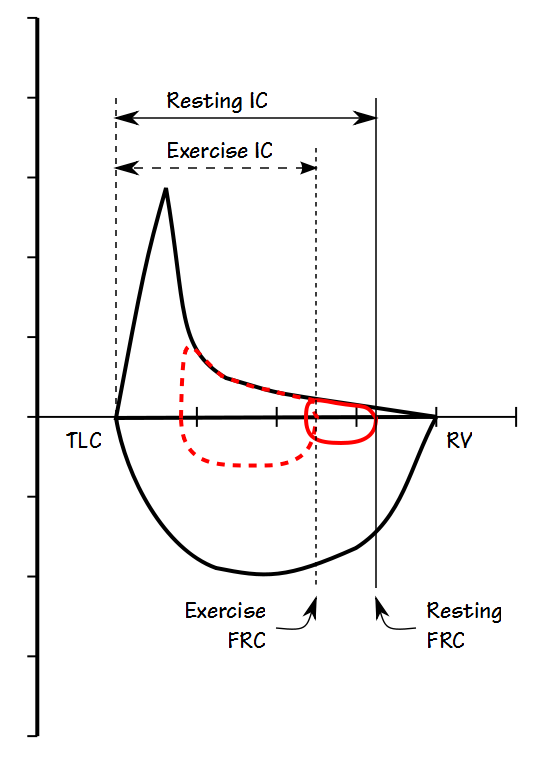
One of the hallmarks of COPD is expiratory flow limitation. This can cause hyperinflation and is often reflected in an elevated FRC. It is also an important factor in exercise limitation. When ventilation increases during exercise in an individual with COPD, expiratory flow limitation causes the tidal volume and FRC to shift towards higher lung volumes. FRC is difficult to measure during exercise so this usually observed by measuring Inspiratory Capacity (IC).
COPD patients who don’t show a significant change in their FEV1 can respond to bronchodilators by becoming less expiratory flow-limited and when this happens their FRC decreases. Bronchodilator response in these individuals can therefore be assessed by measuring pre- and post-bronchodilator FRC or IC. At present there appears to be a consensus that an increase in IC or decrease in FRC of at least 0.30 liters or 12% should be considered to be a significant response.
At first glance it would seem that measuring FRC would be the most accurate way to assess post-BD improvement but there are a number of criticisms towards measuring FRC in patients with severe airway obstruction. Under some circumstances the FRC measured by plethysmography can be falsely elevated in individuals with COPD. It could also be argued that gas trapping can cause FRC to be underestimated when it is measured by helium dilution or nitrogen washout. For these reasons IC is probably a more reliable indication of a change in FRC which is also convenient because IC can be measured as part of an SVC maneuver more quickly than FRC and with simpler equipment.
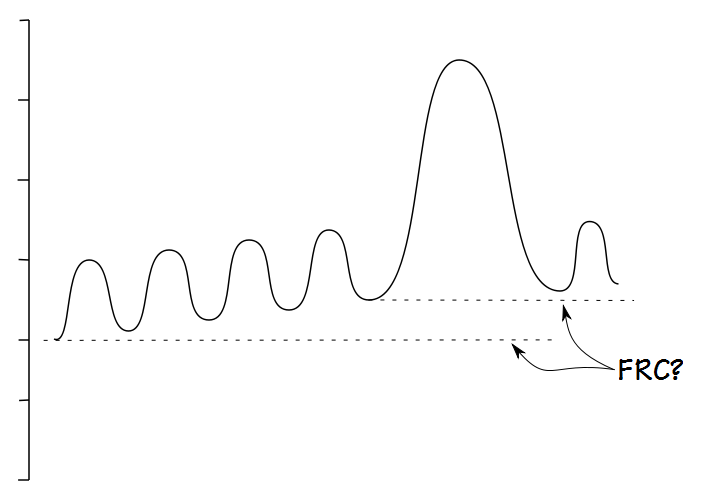
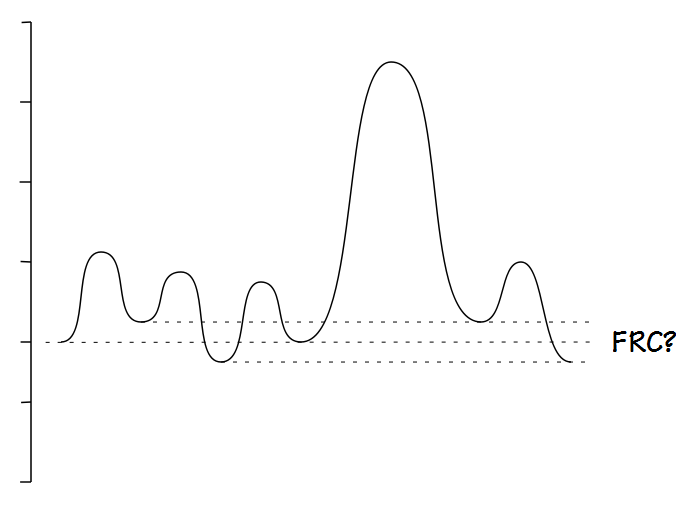
This doesn’t mean that measuring IC doesn’t require a certain amount of care. IC is difference in volume between FRC and TLC. FRC is defined as the volume of the lung at the end of a normal exhalation and in any individual, not just those with COPD, FRC is a dynamic volume because it is balance point between a number of forces. For this reason determining where the “normal” end of exhalation resides is critical when measuring IC but is also somewhat poorly defined.
It should be relatively obvious that FRC cannot be determined when there is a leak.
It is also fairly obvious that FRC is difficult to determine when an individual is breathing erratically.
The ATS/ERS statement on spirometry states that for a SVC maneuver the subject be “… asked to breathe regularly for several breaths until the end-expiratory lung volume is stable (this usually requires at least three tidal manoeuvres)”. Other than this there is no specific definition for what determines a stable end-exhalation and like many other things that are “obvious” a stable exhalation is difficult to define.
Until we have a better definition of what constitutes a stable end-exhalation the best that we can do is to “eyeball” the results and try to choose results that appear to have good quality. This can be a problem because I’ve seen several spirometry systems, usually the simpler and less-expensive ones, where only numerical SVC results are displayed. When the SVC is shown graphically sometimes only the SVC component is displayed and sometimes the display is low resolution and small details cannot be seen. For all of these reasons an accurate end-exhalation level can only be determined when the tidal breathing component of the SVC test is displayed in its entirety and at a high enough resolution for small details to be evident.
Even when the tidal breathing component of the SVC is displayed correctly it is also necessary to be able to edit the end-exhalation level. I suspect that most spirometry systems do a reasonably good job of averaging the end-exhalation volume for the IC measurement, but that doesn’t mean they are always correct. Without the ability to edit SVC results there will be times where an adequate SVC maneuver will need to be discarded solely because of an error in the algorithm for determining end-exhalation.
Like other lung volumes, the highest IC is not necessarily the best or most accurate one and for this reason the IC maneuver should be performed several times and an average taken from the most repeatable results. The ATS/ERS statement on spirometry says that FVC and FEV1 are repeatable when results are within 0.15 L (0.10 L if the FVC is less than 1.0 liter) and it seems to be reasonable to expect the same level of repeatability from acceptable IC maneuvers.
Not all individuals with COPD will show post-bronchodilator improvements in IC or FRC. Research indicates that improvements tend only to be seen in individuals with expiratory flow limitation and hyperinflation. There is a general association between COPD severity and expiratory flow limitation but there are no simple methods for determining expiratory flow limitation and it is unclear how many patients with COPD who don’t show an improvement FEV1 will show one in IC or FRC. For these reasons I think that the best approach at present is for all patients with COPD to have their IC measured as part of their pre- and post-bronchodilator spirometry.
Measuring IC as well as regular spirometry will increase the amount of time spent testing a patient with COPD and it cannot be billed separately. Nevertheless, knowing whether or not a patient benefits from the use of a bronchodilator even if their FEV1 does not improve is valuable clinical information and should be considered to be a standard part of the care of an individual with COPD.
References:
Brusasco V, Crapo R, Viegi G. ATS/ERS Task Force: Standardisation of lung function testing. Standardisation of spirometry. Eur Respir J 2005; 26: 319-338.
Brusasco V, Crapo R, Viegi G. ATS/ERS Task Force: Standardisation of lung function testing. Interpretive strategies for lung function tests. Eur Respir J 2005; 26: 948-968.
Duranti R, Filippelli M, Bianchi R, Romagnoli I, Pellegrino R, Brusasco V, Scano G. Inspiratory capacity and decrease in lung hyperinflation with albuterol in COPD. Chest 2002; 122: 2009-2014.
O’Donnell DE. Assessment of bronchodilator efficacy. Is spirometry useful? Chest 2000; 117: 42S-47S.
O’Donnell DE, Forkert L, Webb KA. Evalution of bronchodilator response in “irreversible” emphysema. Eur Respir J 2001; 18: 914-920.
Pellegrino R, Rodarte JR, Brusasco V. Assessing the reversibility of airway obstruction. Chest 1998; 114: 1607-1612.
Taube C, Lehnigk B, Paasch K, Kirsten DK, Jorres RA, Magnussen H. Factor analysis of changes in dyspnea and lung function parameters after bronchodilation in chronic obstructive pulmonary disease. Amer J Respi Crit Care Med 2000; 162: 216-220.

PFT Blog by Richard Johnston is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.